The First Inactivated Enterovirus Type 71 Vaccine (Human Diploid Cell) All Over the World
After 8 years’ dedicated work, EntroVac?, the first vaccine against HFMD caused by Enterovirus Type (EV-71) was approved by CFDA on December 3nd, 2015.
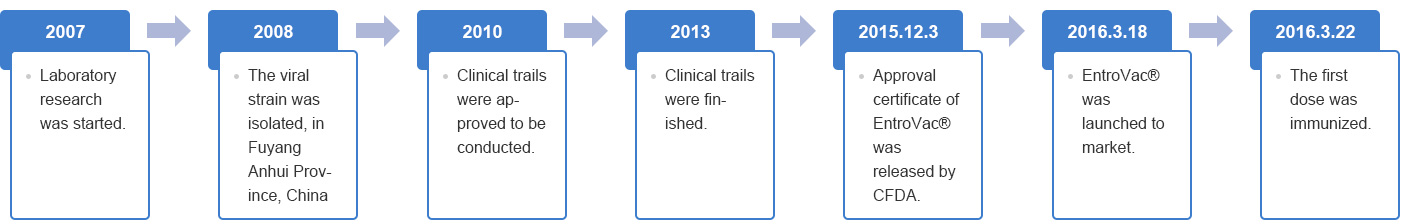
Development milestone:
The Innovative Vaccine from Concept to Practice
Utilizing domestic primate resources, IMBCAMS invented neonatal monkey model to explore pathogenic mechanism of EV-71 and to evaluate the efficacy of EntroVac?.Immune response mechanism induced by EntroVac? was studied in clinical trials. A multi-center phase III clinical trial involving 12,000 subjects at 6 to 71 months of age sufficiently proved the safety and efficacy of EntroVac?. Over two course of epidemic seasons, efficacy of the vaccine reached 97.3%
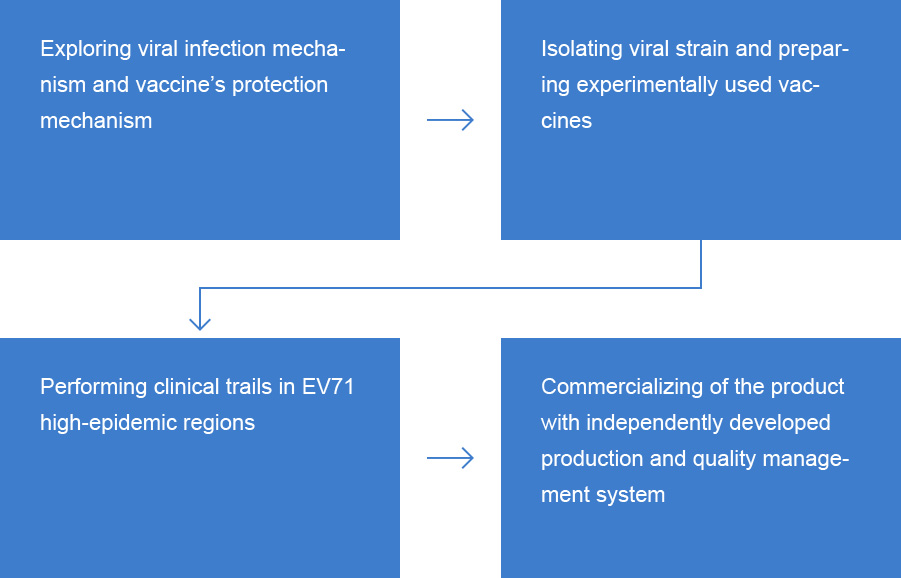
The Persistent Research Provides the World Powerful Tools in Controlling HFMD
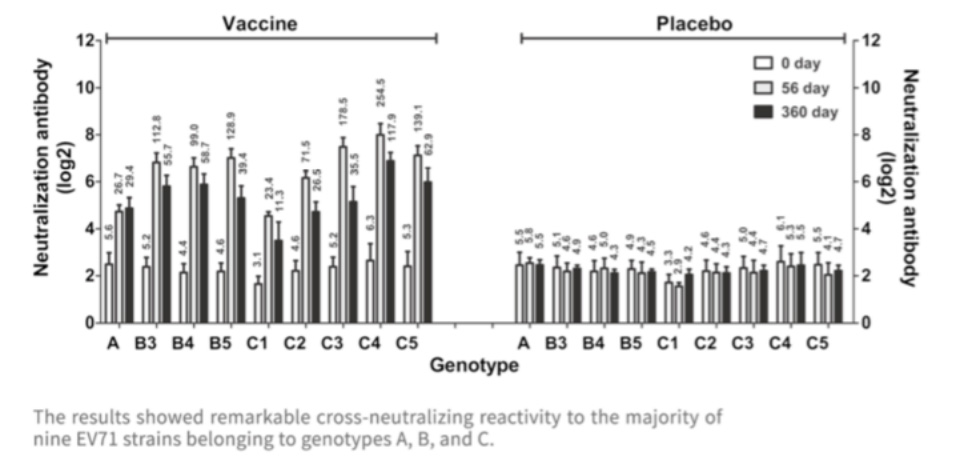
The EntroVac? developed by IMBCAMS is derived from the C4 subtype of EV-71, but the cross-neutralization test suggested that the vaccine could protect against most circulating EV-71 strains in different countries.
At present, scientists in IMBCAMS are pushing forward the research on other major pathogens of HFMD such as Coxsackie virus A16.
In the future, the prospective research of combined vaccines containing different inactivated enteroviruses will provide a stronger strategy in preventing HFMD.


用户登录
还没有账号?
立即注册